
Sirinart Ananvoranich
Professor
Biochemistry
Ph.D. (Concordia)
anans@uwindsor.ca
253-3000 Ext. 3550
275-3 Essex Hall
Research Area: Biochemistry
More Info: Personal homepage

Tricia Breen Carmichael
Professor
Inorganic/Materials
Ph.D. (University of Windsor)
tbcarmic@uwindsor.ca
253-3000 Ext. 3538
373-6 Essex Hall
Research Area: Materials Chemistry
More Info: Personal homepage

Philip J. Dutton
Associate Professor
Organic Chemistry
Ph.D. (Victoria)
dutton@uwindsor.ca
253-3000 Ext. 3526
273-2 Essex Hall
Research Area: Physical Organic
More info: Personal homepage

S. Holger Eichhorn
Professor
Organic/Physical
Ph.D. (Bremen Germany)
eichhorn@uwindsor.ca
253-3000 Ext. 3990
375-4 Essex Hall
Research Area: Materials/Organic/Polymer/Chemistry
More info: Personal Homepage

James R. Green
Professor
Organic
Ph.D. (Waterloo)
jgreen@uwindsor.ca
253-3000 Ext. 3545
358 Essex Hall
Research Area: Organic Chemistry
More info: Personal Homepage

Scott G. Harroun
Assistant Professor
Biosensors/Nanomaterials
Ph.D. (Université de Montréal)
Scott.Harroun@uwindsor.ca
(519)-253-3000 Ext. 3535
275-D Essex Hall
Research Area: Biosensors and Nanomaterials
More info: Personal Homepage

Samuel A. Johnson
Professor
Inorganic/Materials
Ph.D. (University of British Columbia)
sjohnson@uwindsor.ca
253-3000 Ext: 3769
329 Essex Hall
Research Area: Transition Metal Chemistry & Materials
More info: Personal homepage

Tranum Kaur
Academic Ancillary Staff-Learning Specialist III
MMB Program Coordinator
tranum@uwindsor.ca
253-3000 Ext. 3570
251 Essex Hall

Drew Marquardt
Associate Professor
Physics (Biophysics)
Ph.D. (Brock University)
Research: Biomaterials
Drew.Marquardt@uwindsor.ca
+1-(519)-253-3000 Ext. 3537
275-3 Essex Hall
Research Area: Biomembranes
More info: Personal homepage

Scott Mundle
Associate Professor
Analytical
Ph.D. (University of Toronto)
smundle@uwindsor.ca
Phone: (519)-253-3000 Ext. 3755
Fax: (519) 971-3616
GLIER Rm: 223
Research Area: Environmental Chemistry/Geochemistry
More info: Personal homepage

Kenneth Ng
Professor
Biochemistry Ph.D. (Stanford University)
kksng@uwindsor.ca
253-3000 Ext. 3574
375-5 Essex Hall
Research Area: Structural Biology
More info: Personal homepage

Siyaram Pandey
Distinguished University Professor
Biochemistry Ph.D. (J.N.U., New Delhi)
spandey@uwindsor.ca
253-3000 Ext. 3701
277-1 Essex Hall
Research Area: Cell Death
More info: Personal homepage

Jeremy Rawson
Professor
Inorganic/Materials
Ph.D. Inorganic Chemistry (Durham University, UK)
jmrawson@uwindsor.ca
253-3000 Ext: 3700
387=A Essex Hall
Research Area: Inorganic and Molecular Materials
More info: Personal Homepage

Simon Rondeau-Gagné
Associate Professor
Organic/Materials
Ph.D. (Université Laval)
Simon.Rondeau-Gagne@uwindsor.ca
Phone: (519)-253-3000 Ext. 3556
Fax: (519)-973-7098
375-1 Essex Hall
Research Area: Organic Materials
More info: Personal homepage

John F. Trant
Associate Professor
Organic/Materials
Ph.D. (University of Ottawa)
j.trant@uwindsor.ca
Phone: (519)-253-3000 Ext. 3528
Fax: (519)-973-7098
373-4 Essex Hall
Research Area: Biopolymeric materials and (Bio)organic synthesis
More info: Personal homepage

Yufeng Tong
Associate Professor
Biochemistry
Ph.D. (Chinese Academy of Sciences, Beijing)
ytong@uwindsor.ca
519-253-3000 Ext. 3551
Rm. 355 Essex Hall
Research Area: Experimental Biochemistry
More info: Personal homepage

Panayiotis O. Vacratsis
Professor
Biochemistry
Biochemistry Ph.D. (Michigan State)
vacratsi@uwindsor.ca
253-3000 Ext: 3541
263 Essex Hall
Research Area: Cell Signaling and Proteomics
More info: Personal homepage

Nick Vukotic
Assistant Professor
Inorganic/Materials
Ph.D. (University of Windsor)
nvukotic@uwindsor.ca
253-3000 Ext. 3572
375-3 Essex Hall
Research Area: Materials Chemistry
More info: Personal Homepage

Jichang Wang
Professor
Cross-Appointed: Department of Physics
Physical Chemistry Ph.D. (Copenhagen)
jwang@uwindsor.ca
253-3000 Ext: 3540
373-5 Essex Hall
Research Area: Non-linear Chemical Dynamics
More info: Personal homepage

Zhuo Wang
Associate Professor
Polymer and Materials Chemistry Ph.D.
(University of North Carolina at Chapel Hill) zhuowang@uwindsor.ca
253-3000 Ext: 4235
375-6 Essex Hall
Research Area: Polymer & Materials Chemistry
More info: Personal homepage
ADJUNCT, EMERITUS & RETIRED FACULTY
 Dr. Ricardo Aroca
Dr. Ricardo Aroca
Physical and Analytical Chemistry
Ph.D. (Moscow State), D.Sc.(Leningrad)
g57@uwindsor.ca
University Professor
Cross-Appointed: Department of Physics
253-3000 Ext. 3528
373-4 Essex Hall
Personal Homepage
RESEARCH INTERESTS:
- Single-Molecule Detection (SMD) using surface-enhanced resonance Raman scattering and Langmuir-Blodgett monolayers. The development to this new approach to SMD was published in Analytical Chemistry2001, 73, 3674, followed by two new reports and recently the first report of overtone and combinations in SMD was also published in Analytical Chemistry 2003, 75, 1918-1923). In this work the Langmuir-Blodgett (LB) technique has been used to obtain the surface-enhanced resonance Raman scattering (SERRS) spectra of single dye molecules in the matrix of a fatty acid. Mixed monolayers of the dye material have been fabricated on silver islands substrates with a concentration of the probed molecule of one molecule per micron square. Supported by an NSERC research grant.
- Nanostructures for Surface-Enhanced Spectroscopy. Surface enhanced-vibrational spectroscopy, surface enhanced fluorescence and nanostructure fabrication with appropriate optical properties to produce enhanced optical fields. Supported by an NSERC research grant.
- With the support of General Motors of Canada we are starting a program on hydrogen storage materials. In this work we shall systematically explore the nature of chemical bonding in a series of hydrides using real-time temperature programmed vibrational spectroscopy in a wide range of temperature. We also examine the complex chemical hydrides using high level ab initio theoretical calculations. One of the main goals of this research work is to optimize the hydride composition to improve the kinetics of hydrogen sorption in ABH4 compounds, where A is alkaline or mixed alkaline elements and B is an sp elements such as B, Al or Ga. Supported by a CRD NSERC grant
SELECTED PUBLICATIONS:
- P.N.W. Pieczonka and R.F. Aroca, Inherent Complexities of Trace Detection by Surface-Enhanced Raman Scattering. ChemPhysChem, 2005, p-1-13. Minireviews, Published Online: 18 Nov 2005.
- R.F. Aroca, R.A. Alvarez-Puebla, N. Pieczonka, S. Sanchez-Cortez, and J.V. Garcia-Ramos, (Review) Surface-enhanced Raman scattering on colloidal nanostructures. Advances in Colloid and Interface Science, 2005. 116: p. 45-61.
- Goulet, P.J.G., N.P.W. Pieczonka, and R.F. Aroca, Mapping single-molecule SERRS from Langmuir-Blodgett monolayers on nanostructured silver island films. Journal of Raman Spectroscopy, 2005. 36(6/7): p. 574-580.
- Alvarez-Puebla, R.A., E. Arceo, P.J.G. Goulet, J.J. Garrido, and R.F. Aroca, Role of Nanoparticle Surface Charge in Surface-Enhanced Raman Scattering. Journal of Physical Chemistry B, 2005. 109(9): p. 3787-3792.
- Aroca Ricardo, F., J.G. Goulet Paul, S. Dos Santos David, Jr., A. Alvarez-Puebla Ramon, and N. Oliveira Osvaldo, Jr., Silver nanowire layer-by-layer films as substrates for surface-enhanced Raman scattering. Analytical Chemistry, 2005. 77(2): p. 378-82.
Professor Emeritus
Adjunct Professor
Professor
Biochemistry/Physical
Ph.D (Australian National University)
RESEARCH INTERESTS:
Central to understanding the chemistry of cells is elucidating the chemistry of important biomolecules and their interactions. A class of biomolecules of particular interest is enzymes; molecules that catalyse the broad range of reactions essential for life. In addition to the fundamental knowledge to be learned, this interest is due in part to the medical and industrial benefits to be obtained. For example, the aim of therapeutic drugs is often to enhance or inhibit the function of a specific enzyme. Computational chemistry uses computers to model the chemistry and reactions of chemical systems. Such approaches are used, for example, to study problems that may be too difficult to study experimentally or to provide insight into observed phenomena. Our research group uses the methods of computational and theoretical chemistry to investigate the chemistry and reactions of various biomolecules, in particular biochemical catalysts. Brief overviews of some various areas studied by our group are described below.
PUBLICATIONS:
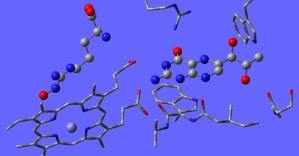 The Chemistry of cofactors:
The Chemistry of cofactors:
Many enzymes require essential cofactors in order to perform their catalytic function. Such species often act as asource of functional groups or electrons. Their behaviour can differ between enzymes due to chemical variations in their binding sites which influence the structure and properties of the cofactor, tuning it to its role in the given enzyme. However, how such differences in the binding site affect the cofactors are often unknown. Modeling cofactor binding can thus provide greater insight into their roles in enzymes.
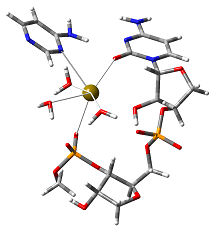 Modeling active sites:
Modeling active sites:
Enzymes exploit cooperative effects of chemical interactions and environmental effects in order to catalyse reactions. In addition to investigating the actual catalytic mechanism, by modeling the structure of the active site one can study the role of individual enzyme-substrate interactions in the mechanism. Such studies not only provide deeper insight into how an enzyme actually functions, but also into, for example, what may be the consequences of a particular mutation. A focus of our research is the mechanisms and properties of ribo- and DNAzymes (catalytic nucleic acids) and novel protein metalloenzymes.
 DNA multiplexes:
DNA multiplexes:
During replication, single stranded DNA at the end of chromosomes are progressively shortened, thus regulating cell division. In cancer cells, however, certain enzymes are capable of repairing these ends, leading to immortality. It is thought that these single strands of DNA can form intra- or inter-strand complexes, leading to the formation of variously stacked multiplexes, thus making themselves unavailable to repair enzymes. As a result, there is considerable interest in developing therapeutic drugs that specifically bind such complexes. Hence, better understanding of the structure and properties of such multiplexes could lead to the development of new therapies.
SELECTED PUBLICATIONS:
- Dokainish, H.; Gauld, J.W.* (2013) An MD and QM/MM study on the Catalytic Reductase Mechanism of Methionine Sulfoxide Reductase A (MsrA): Formation and Reduction of a Sulfenic Acid. Biochemistry 52, 1814-1827.
- Bushnell, E.A.C.; Jamil, R.; Gauld, J.W.* (2013) Insights into the Chemistry of Lipoxygrnases (LOXs): A Computational Investigation Into The Catalytic Mechanism of 8R-LOX. J. Biol. Inorg. Chem. 18, 343-355.
- Bushnell, E.A.C.; Fortowsky, G.B.; Gauld, J.W.* (2012) Iron-Oxo Species and the Oxidation of Imidazole: Insights into the Mechanism of OvoA and EgtB. Inorg. Chem. 51, 13351–13356.
- Huang, W.J.; Gauld, J.W.* (2012) Tautomerization in the UDP-Galactopyranose Mutase Mechanism: A DFT-Cluster and QM/MM Investigation. J. Phys. Chem. B 116, 14040–14050.
- Ion, B.F.; Bushnell, E.A.C.; De Luna, P.; Gauld, J.W.* (2012) An MD and QM/MM Study on Ornithine Cyclodeaminase (OCD): A Tale of Two Iminiums. Int. J. Mol. Sci., 13, 12994-13011.
- Bushnell, E.A.C.; Gauld, J. W.* (2012) An Assessment of Standard, Hybrid, Meta and Hybrid-Meta GGA Density Functional Theory Methods for Open-Shell Systems: the Case of the Non-Heme Iron Enzyme 8R–LOX. J. Comput. Chem. 34, 141-128.
- Huang, W.J.; Gherib, R.; Gauld, J.W.* (2012) An Active Site Water Broadens Substrate Specificity in S-Ribosylhomocysteinase (LuxS): A Docking, MD, and QM/MM Study. J. Phys. Chem. B, 116, 8916-8929.
- Bushnell, E.A.C.; Huang, W.J.; Llano, J.; Gauld, J.W.* (2012) Molecular Dynamics Investigation into Substrate Binding and Identity of the Catalytic Base in the Mechanism of Threonyl-tRNA Synthetase. J. Phys. Chem. B, 116, 4205-5212.
- Almasi, J.; Bushnell, E.A.C.; Gauld, J.W.* (2011). A QM/MM-based Computational Investigation on the Catalytic Mechanism of Saccharopine Reductase. Molecules, 16, 8569-8589.
Professor Emeritus

RESEARCH INTERESTS:
Thrombosis or clotting, is the cause of 50% of deaths in North America. However, blood coagulation is essential in arresting hemorrhaging. Thus, there must be a delicate balance betwen these processes. When this balance is disturbed, patients suffer from hemphilia, strokes, heart attacks and cardiovascular disease. Heparin, a negatively charged polysaccharide, is a potent anticoagulant which ihnhibits blood clotting. The protein platelet factor 4 plays a key role in blood coagulation.
Our major focus is on the application of nuclear magnetic resonance spectroscopy to study the structure and function of platelet factor 4 and other platelet proteins, in particular the novel bovine platelet plasmin inhibitor. Nuclear magnetic resonance techniques are used to examine the environments of individual amino acids in proteins. The assignment of the resonance requires the applications of protein chemical techniques. These studies enable us to ascertain the role of specific amino acids in the structure of the platelet protein, to determine their contributions to heparin binding, and to probe protein conformation.
Our interests also extend to thymosin alpha 1, a peptide which is used in clinical trials for the treatment of hepatitis b, and ArsC, a protein which confers arsenate resistance to cells and is homologous to the multidrug resistance proteins. The elucidation of the structure of these peptides and proteins provides insights into their function.
SELECTED PUBLICATIONS:
- Baldwin, J.S., Lee, L., Leung, T.K., Muruganandam, A., Mutus, B. Identification of the Site of Non- enzymatic Glycation of Glutathione Peroxidase: Rationalization of the Glycation-Related Catalytic Alterations on the Basis of 3-D Protein Structure., Biochimica et Biophysica Acta, in press (1994).
- Veenstra, T.D., Lee, L. NMR Investigation of the Position of Histidine-119 in the Complex of Ribonuclease A with Uridine Vanadate in Solution, Biophysical J. 67, 331-335 (1994).
- Talpas, C.J., Lee, L. Comparative Studies of the Interaction of Human and Bovine Platelet Factor 4 with Heparin Using Histidine NMR Resonances as Spectroscopic Probes. J. Protein Chemistry 12, 303-309 (1993).
- Cederholm, M.T., Stuckey, J.A., Doscher, M.S., Lee, L. Histidine pKa Shifts Accompanying the Inactivating D121N Substitution in a Semisynthetic Bovine Pancreatic Ribonuclease, Proc. Natl. Acad. Sci. USA 88, 8116-8120 (1991).
- Talpas, C.J., Walz, D.A., and Lee, L. 1H NMR Studies of Bovine Platelet Factor 4: Histidine Assignments and Interactions with Heparin, Biochimica et Biophysica Acta 1078, 208-218 (1991).
- Kim, H.S., Lee, L., and Evans, D.R. Identification of the ATP Binding Sites of the Carbamyl Phosphate Synthetase Domain of CAD, the Multifunctional Protein that Initiates Pyrimidine Biosynthesis in Mammalian Cells, Biochemistry 30, 10322-10329 (1991).
Professor Emeritus

SUPRAMOLECULAR CHEMISTRY
We are a supramolecular chemistry group with a keen interest in mechanically interlocked molecules (MIMs). Our chemistry involves developing new supramolecular templating motifs for the formation of interpenetrated host-guest species called pseudorotaxanes and their conversion into permanently interlocked molecules such as rotaxanes and catenanes.
One of our primary goals is to create organized solid state materials that incorporate these artificial molecular switches and machines. We are attempting to achieve this higher level of molecular organization by placing the dynamic molecular components of a MIM (e.g. rotation or translation) into either the pores of a metal–organic framework (MOF) material or the core structure of a mesomorphic liquid crystalline material.
Professor Emeritus
Professor Emeritus

RESEARCH INTERESTS: Redox Signaling in Vascular Cells
Nitric oxide (NO) and hydrogen sulfide (H2S) are important signaling molecules produced in the body. They play major roles in the function as well as dysfunction of the nervous system, immune system and the circulatory system. NO and H2S are very reactive compounds that react with each other as well as with oxygen and biomolecules to form both stable and unstable derivatives that help mediate their physiological and pathological effects. However, whether the cellular processes initiated by NO and H2S are independent or augmented or counteracted by each other, is poorly understood.
Dr. Mutus' research investigates the interplay of NO and H2S signaling in animal, yeast and plant cells. The research group develops new fluorescent probes to monitor the levels of thiols, S-nitrosothiols, NO and H2S to identify their protein adducts. The probes are used in live cells to study the activity and function of target enzymes as well as determining whether either molecule affects the storage or release of the other.
Research Interest/Expertise Keywords: enzymology, protein structure-function, redox signaling, dynamic microscopy, cell biology, intravital fluorescent probes, nano sensors for thiols, nitric oxide, S-nitrosothiols and NOx, platelet biochemistry, flow devices, environmental sensor development, mitigation of environmental phosphate.
Professor Emeritus
Professor Emeritus

Applied Enzymology & Environmental Biotechnology. Enzyme-based methods are being developed as an alternative strategy for industrial wastewater treatment. This work is done in close collaboration with a campus group in environmental engineering using isolated enzymes, either free in solution or in immobilized form. In some cases, enzymatic methods of analysis are developed to complement the spectroscopic and chromatographic ones that are used in process development and modeling. We have broadened the scope of applicability by developing two, separate chemical "front-ends" for the enzyme-based process. Thus, zero-valent metal reduction converts nitro-aromatics into anilines. Similarly, Fenton-type chemistry converts aromatics of the BTEX, PAH, PCB and dioxin classes into phenolics. The products of both of these chemical processes are readily treated by the enzyme-based process. The goal of this project is to design cost-effective reactors for treatment of selected target streams to yield effluents in compliance with environmentally-acceptable discharge limits. Knowledge of protein chemistry, enzyme mechanisms and inactivation, spectroscopic and chromatographic methods, environmental (organic) chemistry and process monitoring, optimization and control will be required or acquired in varying degrees. Students gaining experience in the above areas would be well-suited for employment related to the chemical aspects of environmental biotechnology (analytical techniques, purification, characterization, utilization, downstream processing, production) and this would be complementary to the engineering and molecular biological aspects of the field.
SELECTED PUBLICATIONS:
- M. Mani Biswas, N. Biswas, K.E. Taylor and J.K. Bewtra (2007). Enzymatic treatment of sulfonated aromatic amines generated from reductive degradation of reactive azo dyes. Water Environment Research 79, 351 – 356.
- S. Dasgupta, K.E. Taylor, N. Biswas and J.K. Bewtra (2007). Inactivation of enzyme laccase and role of co-substrate oxygen in enzymatic removal of phenol from water. Water Environment Research 79, 858 – 867.
- J. Patapas, M. Mousa Al-Ansari, K.E. Taylor, J.K. Bewtra and N. Biswas (2007). Removal of dinitrotoluenes from water via reduction with iron and peroxidase-catalyzed oxidative polymerization: a comparison between Arthromyces ramosus peroxidase and soybean peroxidase. Chemosphere 67, 1485 – 1491 (doi:1010.1016/j.chemosphere.2006.12.040).
- J.P. Ghosh, K.E. Taylor, J.K. Bewtra and N. Biswas (2008). Laccase-catalyzed removal of 2,4-dimethylphenol from synthetic wastewater: effect of polyethylene glycol and dissolved oxygen. Chemosphere 71, 1709 – 1717 (doi: 10.1016/j.chemosphere.2008.01.002).
- B. Saha, K. E. Taylor, J. K. Bewtra, N. Biswas (2008). Laccase-catalyzed removal of diphenylamine from synthetic wastewater. Water Environment Research 80, 2118 – 2124 (doi: 10.2175/106143008X304712).
- A Steevensz, M. Mousa Al-Ansari, K.E. Taylor, J.K. Bewtra, N. Biswas (2009). Comparison of soybean peroxidase with laccase in the removal of phenol from synthetic and refinery wastewater samples. Journal of Chemical Technology and Biotechnology 84, 761 – 769 (doi: 10.1002/jctb.2109).
- M. Mousa Al-Ansari, A. Steevensz, N. Al-Aasm, K.E. Taylor, J.K. Bewtra, N. Biswas (2009). Soybean peroxidase-catalyzed removal of phenylenediamines and benzenediols from water. Enzyme and Microbial Technology 45, 253 – 260 (doi:10.1016/j.enzymictec.2009.07.004).
- Mohammad Mousa Al-Ansari, A. Steevensz, K. E. Taylor, J. K. Bewtra and N. Biswas (2010). Soybean peroxidase-catalyzed removal of an aromatic thiol, 2-mercaptobenzothiazole, from water. Water Environment Research 82, 2285 – 2289(5); (doi:10.2175/106143010X12681059116617).
- M. Mousa Al-Ansari, Katy Modaressi, K. E. Taylor, J. K. Bewtra and N. Biswas (2010). Soybean peroxidase-catalyzed oxidative polymerization of phenols in coal-tar wastewater: Comparison of additives in enhancing the treatment. Environmental Engineering Science 27, 967 – 975 (doi:10.1089/ees.2010.0143).
- B. Saha, K. E. Taylor, N. Biswas and J. K. Bewtra (2011). Laccase-catalyzed removal of phenol and benzenediols from wastewater. American Society of Civil Engineering’s Practice Periodical, Journal of Hazardous, Toxic and Radioactive Waste Management 15(1), 13 – 20 (online July 26, 2010; doi: 10.1061/(ASCE)HZ.1944-8376.0000050).
- B. Saha, K. E. Taylor, J. K. Bewtra and N. Biswas (2011). Removal of benzene from wastewater via Fenton pre-treatment followed by enzyme-catalyzed polymerization. Water Science and Technology 63.8, 1663 – 1668 (doi: 10.2166/wst2011.331).
- M. Mousa Al-Ansari, B. Saha, S. Mazloum, K.E. Taylor, J.K. Bewtra and N. Biswas (2011). Soybean peroxidase applications in wastewater treatment. In “Soybeans: Cultivation, Uses and Nutrition”, J.E. Maxwell (ed.), Nova Science Publishers, Inc., Hauppauge, NY, ISBN: 978-1-61761 -762-1; Chapter 5, 33 pages.
- A. Steevensz, M. Mousa Al-Ansari, K.E. Taylor, J.K. Bewtra and N. Biswas (2012). Oxidative coupling of various aromatic phenols and anilines in water using a laccase from Trametes villosa and insights into the “PEG effect”. Journal of Chemical Technology and Biotechnology 87, 21 – 32 (published online Oct. 12, 2011; doi: 10.1002/jctb.2734).
- W. Feng, K.E. Taylor, N. Biswas and J.K. Bewtra (2013). Case study: phenolic precipitate utilization to enhance enzymatic wastewater treatment. Canadian Society for Civil Engineering 2013 General Conference (Montreal, May 29 – June 1) Proceedings, paper GEN-121 (6-page extended abstract).
- W. Feng, K.E. Taylor, N. Biswas and J.K. Bewtra (2013). Soybean peroxidase trapped in product precipitate during phenol polymerization retains activity and may be recycled. Journal of Chemical Technology and Biotechnology, in press, accepted 27 Feb., 2013 (7 pages, doi: 10.1002/jctb.4075).
- A. Steevensz, S. Madur, M. Mousa Al-Ansari, K.E. Taylor, J.K. Bewtra, N. Biswas (2013). A simple lab-scale extraction of soybean hull peroxidase shows wide variation among cultivars. Industrial Crops and Products, in press, accepted 25 Mar., 2013 (doi: 10.1016/j.indcrop.2013.03.030).
